The main emissions stemming from a calcination plant, which are usually regulated by local and international laws, are: NOx, CO, dust and SO2. Although the CO2 emissions are not directly regulated, there are clear ambitions among producers to reduce these as well. Other emissions from combustion processes include dioxins, furans, PAHs (polyaromatic hydrocarbons), VOCs (volatile organic compounds) and heavy metals however in this article we’ll focus on the main emissions.
There are two types of emission sources: 1) either emissions are a direct result of input into a calciner (be that feed, fuel or other raw materials) which can be called background emissions, or 2) they are generated by processes or conditions in the calciner itself. Fuel NOx, SO2 or Hg emissions would be typical background emissions in this context, whereas thermal NOx, CO or CO2 and dust/particulate emissions are inherent from the reactions occurring in the calciner or the technologies/equipment used in the process. The emissions prevention and reduction strategy may partly or fully depend on the source of the emission.
There are also two possible pollution prevention strategies: 1) pollution prevention, also called pre-control, or 2) add-on emissions control, or post-control. Pollution prevention are techniques that minimize, reduce or prevent creation of emissions at the source. By carefully designing production processes, using non-emission creating or lower emitting substances and implementing conservation techniques, the generation of air pollutants are eliminated as opposed to abating them after they are created. This could, for example, be the selection of low sulfur fuel instead of heavy fuel oil to reduce the SOx emissions. Post-control techniques reduce pollutants after they have been generated by the process. For example, using an electrostatic precipitator to reduce dust emissions to allowable levels or injection of ammonia to break down NOx would be typical examples of add-on emissions control measures.
Nitrogen Oxides (NOx)
NOx emissions are produced by two primary mechanisms during combustion. One is termed “fuel NOx” and is related to the nitrogen content and species of the fuel and the prevailing combustion conditions. The other mechanism is “thermal NOx” and refers to the chemical formation of NO and/or NO2 from N2 and O2, which occurs at temperature exceeding 1400℃.
For European plants, the Emission Limit Value range for NOx is 50 to 150 mg/Nm³ depending on thermal rating. In urban areas, due to the cumulative effect of NOx from vehicle traffic, lower ELVs are often imposed.
Pre-control measures for NOx
NOx emission come from fuel NOx and thermal NOx formation. Fuel NOx emission originate from some of the nitrogen species in the fuel while thermal NOx will generate when flame temperature goes above 1400℃. The controlling NOx emission technique can focus on either fuel nitrogen concentration or flame temperature or both.
In Circulating Fluidized Bed (CFB) combustion the high solids concentration together with external and internal recirculation of solids ensures a vigorous mixing of the fuel with the combustion air, which leads to a very quick dissipation of energy and thus a very homogenous temperature profile and moderate temperatures throughout the reactor. Therefore, the CFB furnace can inherently control thermal NOx generation. Choosing natural gas with low nitrogen content as fuel for the CFB furnace can significantly reduce the fuel NOx emission further. Some thermal NOx can be generated when a continuously operated pre-heat and pilot burner is used.
Post-control measures for NOx
Selective and non-selective catalytic reduction are technically feasible options to reduce NOx emissions. However, such options should be considered with some caution as the temperature profile in the preheating stages would be affected by injecting NOx reducing agents such as ammonia and/or urea, which may have other detrimental effects for efficiency or product quality.
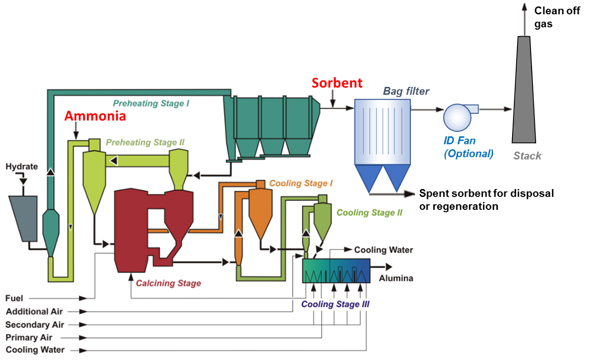
Another option for post-control is the use of a dry sorbent injected upstream of a filter. For example, sodium-based products (such as thermally activated sodium bi-carbonate) readily react with acid gases and can therefore reduce the NOx concentration. However, in the presence of SOx there may be competing mechanisms, so a careful dosing of sorbent becomes critical.
Sulfur Oxides (SOx)
Sulfur oxides, primarily SO2 and sulfur trioxide (SO3), are formed whenever any material that contains sulfur is burned. From 95 to 100% of the total sulfur oxides emissions are in the form of SO2.
SOx emissions limits in the EU are 5-400 mg/Nm³, depending on fuel type and thermal rating. SOx emissions are not only environmentally harmful but can also result in costly corrosion problems in the plant. The risk of SO3 dew point related corrosion can to some extent be mitigated by designing the plant for a higher shell temperature at the affected areas and by operating at higher air/fuel ratios to increase the ESP inlet and stack off gas temperature. Both of these options do however significantly impact on the specific fuel energy consumption, thereby driving up the CO2 emissions as well as incurring higher operating costs.
Pre-control measures for SOx
Sulfur dioxide is formed whenever any material that contains sulfur is burned under oxidizing conditions. Therefore, choosing a lower sulfur content fuel is a fundamental way to reduce SOx emissions. As sulfur in natural gas can be removed easily and economically and the elemental sulfur recovered as a by-product can be sold as a raw material, natural gas contains only trace amount of sulfur. Therefore, the best available technique to control SOx emissions is utilizing low sulfur content fuel, natural gas, which directly reduces the SO2 generation without installation and maintenance, and waste disposal costs. However, in some cases the infrastructure may be lacking for using low S natural gas as fuel.
Post-control measures for SOx
There are effective existing technologies for add-on emissions control for SOx removal, such as dry sorbet injection or various scrubbing technologies. For example, injection of activated sodium bicarbonate in the right dosing ratio can reduce SO2/SO3 emissions by up to 98% and above.
Installing a dry sorbent-based system in a calciner would require installation of an additional system downstream of the existing ESP or baghouse to prevent the recirculation of the spent sorbent within the calcination process, which would obviously contaminate the product alumina. Similarly, the use of a (dry or wet) scrubber would also require installation of a system downstream of the calciner ESP to remove the SOx emissions by spraying water into the gas which causes the SOx to react forming sulfuric acid.
The pro with either of these methods is that they can be highly effective in reducing SOx emissions, but as a downside they do come with a significant investment cost and also require a continuous supply of dosing chemicals and/or disposal of residues.