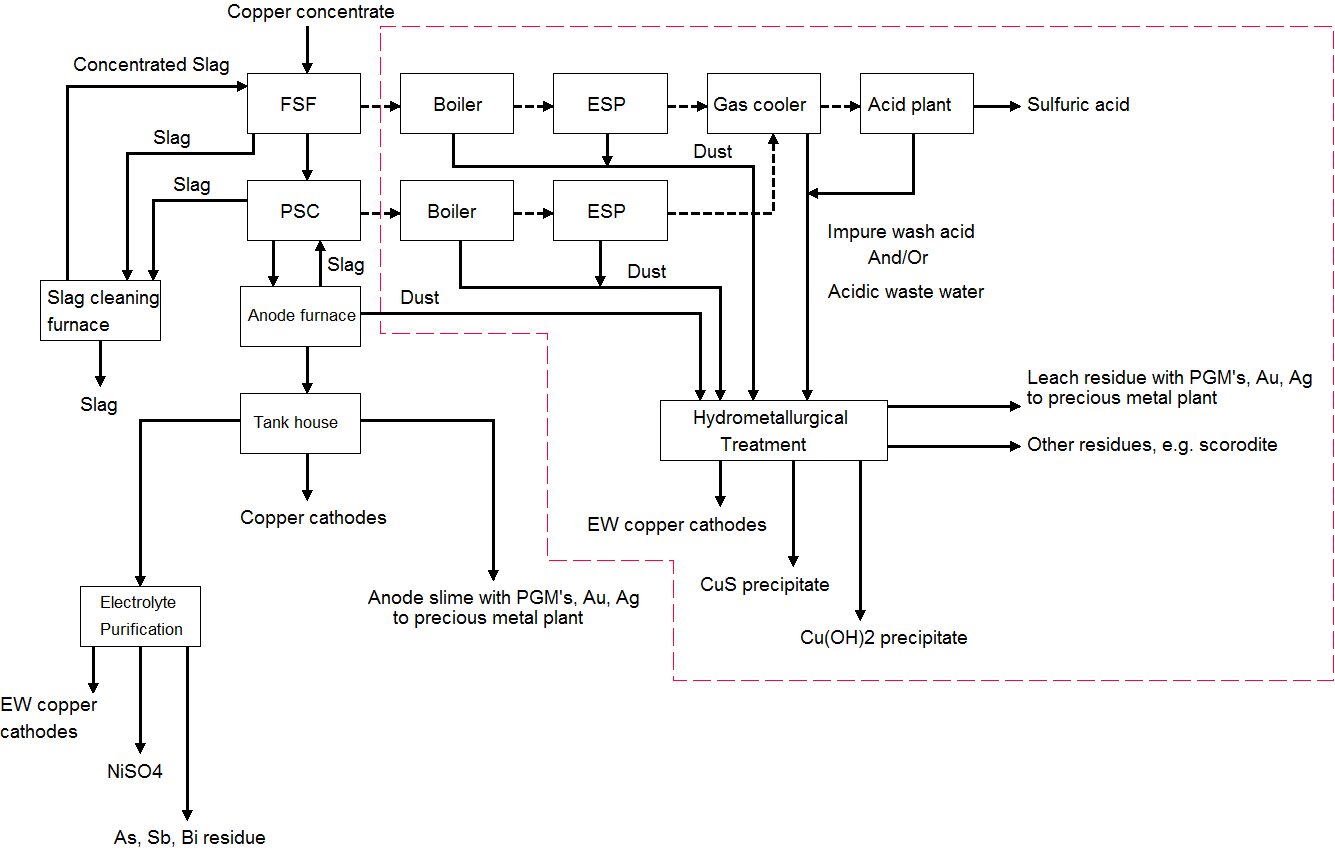
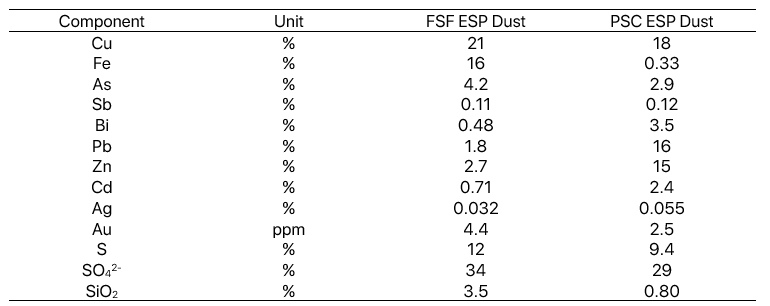
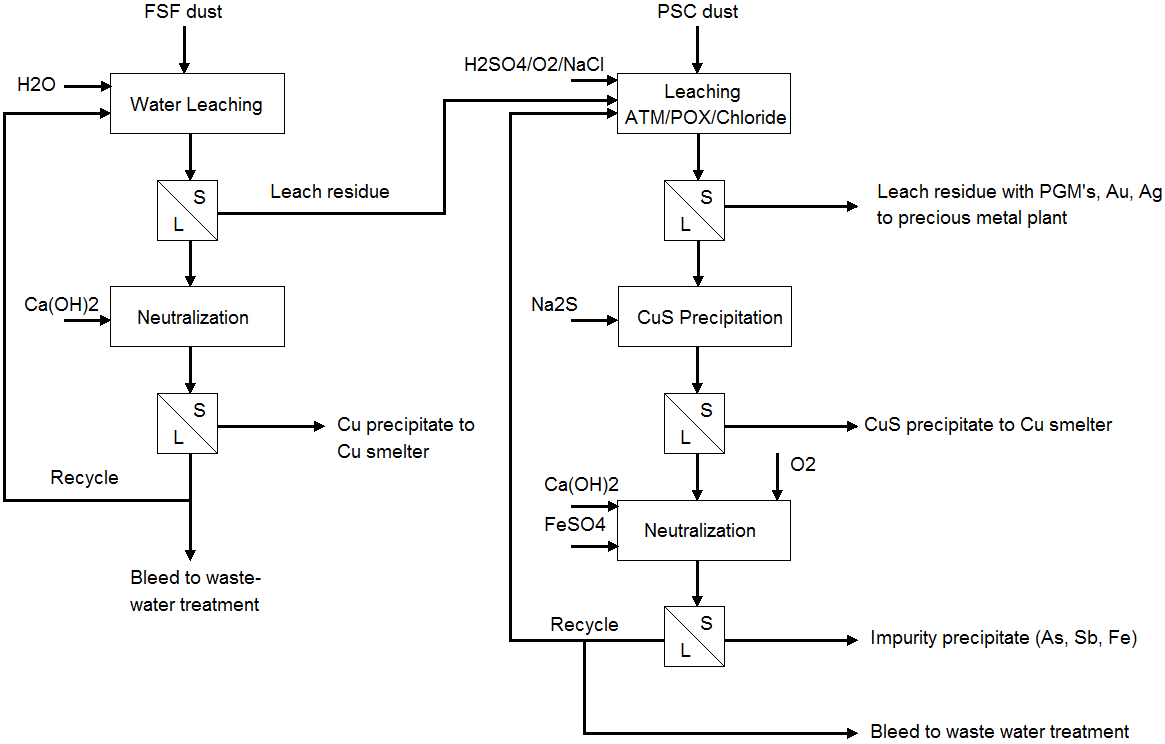
This case gives an overview of hydrometallurgical processes that remove impurity metals and recover copper from flue dust. With impurity metal levels in concentrates increasing, one option to enhance impurity removal from the smelter refinery circuit is hydrometallurgical treatment of smelter flue dust.
The conventional method for controlling flue dust has been recirculation back to the smelter furnace. When impurities start to accumulate in the circuit and there are unacceptable impurity levels in anodes, different solutions need to be used. Even though impurity levels have increased over time, the quality requirements for anodes remain restrictive as higher impurity levels would mean a greater need for solution purification at the electrolytic refinery and more bleed treatment. But as only some of the impurities can be removed with bleed and solution purification, more bleed treatment would mean increased costs for refineries, which cannot be tolerated.
Based on raw material variations and impurity behavior, smelters have become more interested in alternative purification methods. The hydrometallurgical treatment of flue dust is a potential way to control impurities as treating flue dust components externally means impurities do not accumulate, leading to improved impurity control.
Impurity levels can be balanced between flue dust and different slag qualities. Depending on the impurities present, the slag can also be the exit point from a flowsheet with the process tailored for improved recoveries. Based on plant economics, a common feature is that copper needs to be recovered. This can be done in various ways such as directly in copper cathodes, via precipitation, or through crystallization as copper sulfate, which is a powerful coolant when returned to smelter furnaces.
Arsenic, antimony, and bismuth levels in electrolyte also influence electrorefinery efficiency and must be taken into account. The primary reason for hydrometallurgical processing is that there isn’t any other reasonable pyrometallurgical processing method for high-impurity flue dust. A secondary reason is that in some cases the dust contains valuable elements. Especially when the lead and zinc content in flue dust is extremely high and accumulation of these elements in the smelter mass balance needs to be avoided, it is essential to recover these elements using hydrometallurgical processing.
Impurity metal removal and copper recovery can be done, for example, by leaching the flash smelting furnace boiler, converting furnace boiler, and ESP flue dust. Impure wash acid and acidic wastewater from the acid plant can be recycled to the hydrometallurgical plant where it is used for leaching smelter dust (Figure 1).