The increasing electrification of society is fueling a significant surge for rechargeable batteries and the raw materials used in their production. Lithium is one of the key elements enabling electrification, as a typical electric vehicle battery contains several kilograms of lithium. Lithium-ion batteries generally have high energy density with good rechargeability and efficiency compared to other battery types. Main sources of lithium include brine deposits and hard rock minerals, with spodumene being the major mineral source.
The lithium from brine and minerals sources is further refined into various lithium chemical products by the means of hydrometallurgical methods. Typical end products are LiOH, Li₂CO₃ and LiPF₆. Depending on the battery technology, LiOH and Li₂CO₃ are usually used for the cathode and LiPF6 for the electrolyte of the battery.
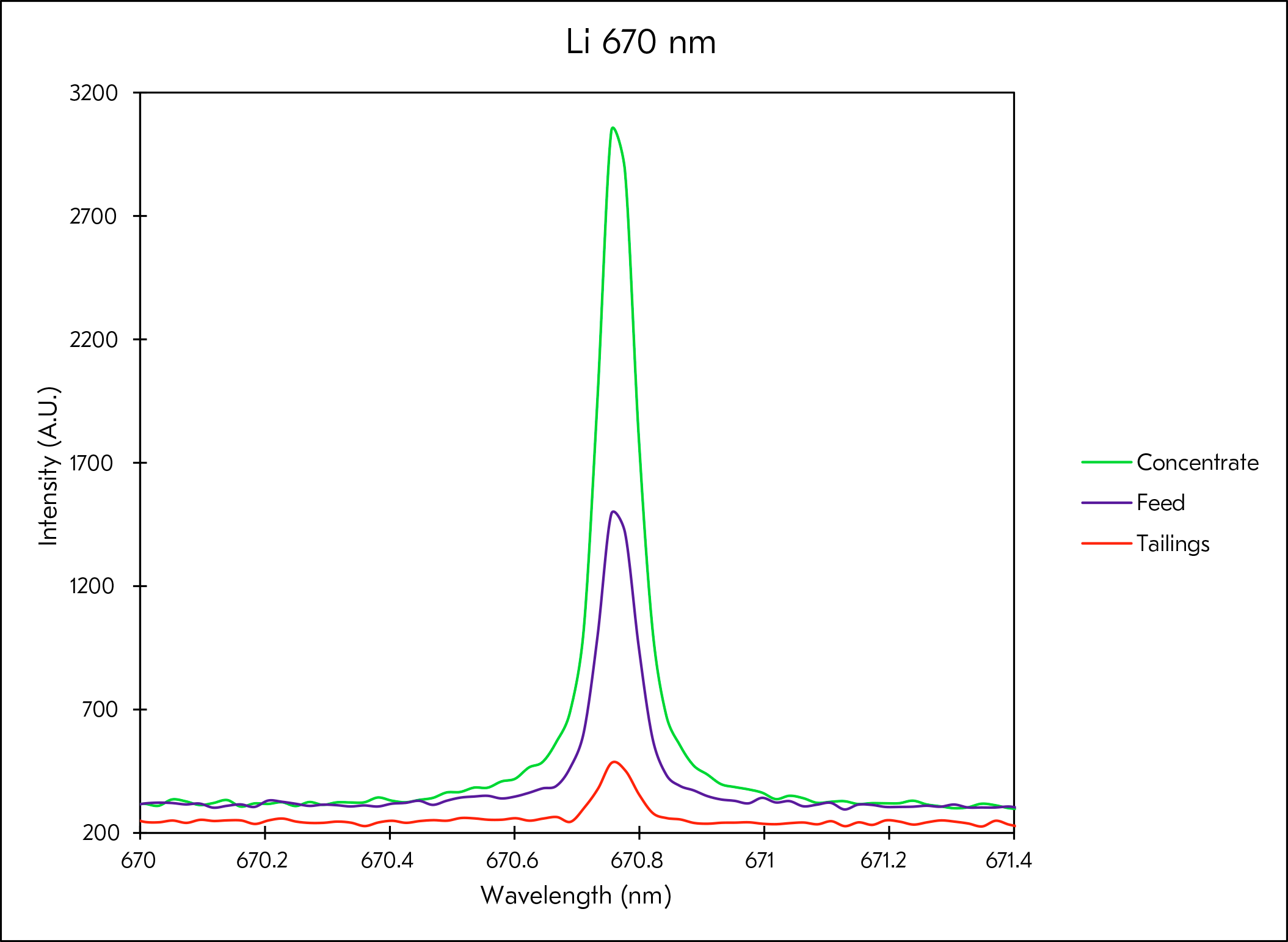
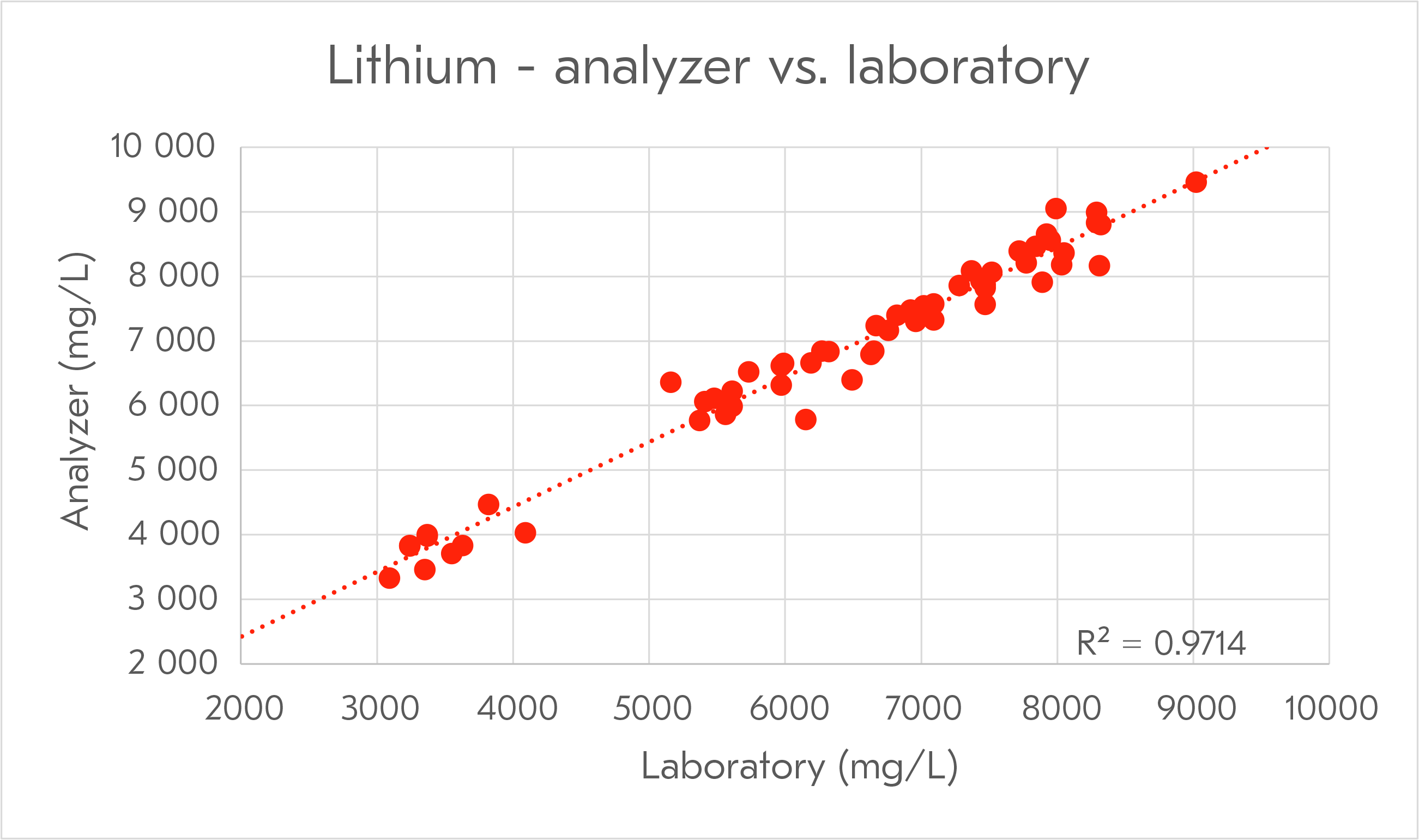
Metso has a long history of developing analyzer and automation solutions in minerals processing and hydrometallurgical processes. Metso’s first commercial Courier® X-Ray Fluorescence analyzer was installed in the 1960's for copper flotation process control. With lithium being one of the first elements in the periodic table, new methods have been developed to analyze it in real-time in both separation and hydrometallurgical processes.