
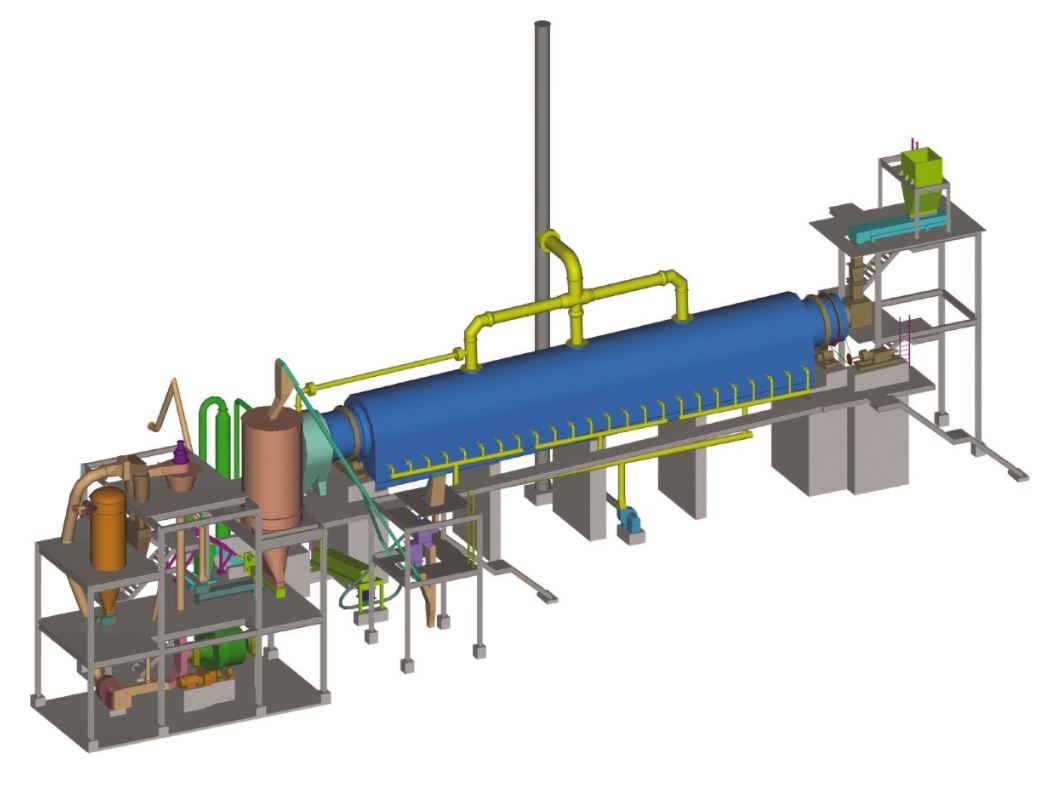
Activated Carbon is used in many industries as an absorber from waste water, to slurries and gases. The absorptive properties of activated carbon are due to the micro-porous structure of each grain resulting into large surface area per unit column of carbon.
In the gold industry, activated carbon is utilized in CIP/ CIL process to transfer dissolved gold from cyanide leached slurry to elution column where gold is desorbed from the carbon.
The stripped carbon will retrain significant quantity of organic and inorganic contaminants (foulants) present in the cyanide slurry. Prior to returning the barren carbon to the process, it is necessary to regenerate the carbon by removing these contaminants (foulants).
Carbon is not selective. It adsorbs other substances very readily. As the cyanide solution percolates through the ore mass it will pick up other elements that it is compatible with. These are often organic in nature. The stripping process regularly leaves these substances behind in the carbon. After a time they restrict the carbon's ability to absorb. Removal of organics from water by AC is predictable and yields important information about the chemicals on the spent AC.
When this occurs, the carbon must be refreshed by having the stray substances removed. To do this the carbon is heated to above 700°C. The problem is that the carbon is flammable at that temperature.
